Hedgehog and Dpp signaling induce cadherin Cad86C expression in the morphogenetic furrow during Drosophila eye development.
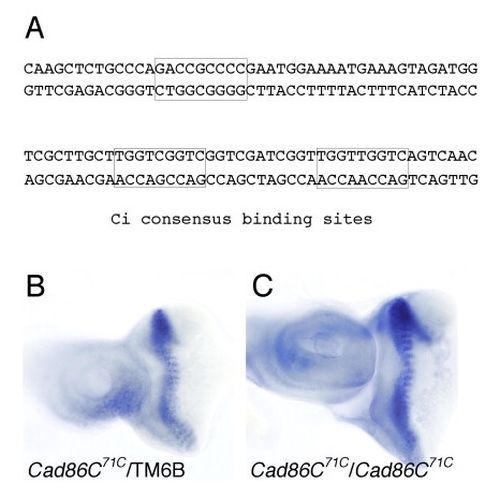
During Drosophila eye development, cell differentiation is preceded by the formation of a morphogenetic furrow, which progresses across the epithelium from posterior to anterior. Cells within the morphogenetic furrow are apically constricted and shortened along their apical-basal axis. However, how these cell shape changes and, thus, the progression of the morphogenetic furrow are controlled is not well understood. Here we show that cells simultaneously lacking Hedgehog and Dpp signal transduction fail to shorten and do not enter the morphogenetic furrow. Moreover, we have identified a gene, cadherin Cad86C, which is highly expressed in cells of the leading flank of the morphogenetic furrow. Ectopic activation of either the Hedgehog or Dpp signal transduction pathway results in elevated Cad86C expression. Conversely, simultaneous loss of both Hedgehog and Dpp signal transduction leads to decreased Cad86C expression. Finally, ectopic expression of Cad86C in either eye-antennal imaginal discs or wing imaginal discs results in apical constriction and shortening of cells. We conclude that Hedgehog and Dpp signaling promote the shortening of cells within the morphogenetic furrow. Induction of Cad86C expression might be one mechanism through which Hedgehog and Dpp promote these cell shape changes.
- Mech. Dev. 2008 Aug 27;125(8):712-28
- 2008
- Developmental Biology
- 18539010
- PubMed
Enabled by:
