Order of lipid phases in model and plasma membranes.
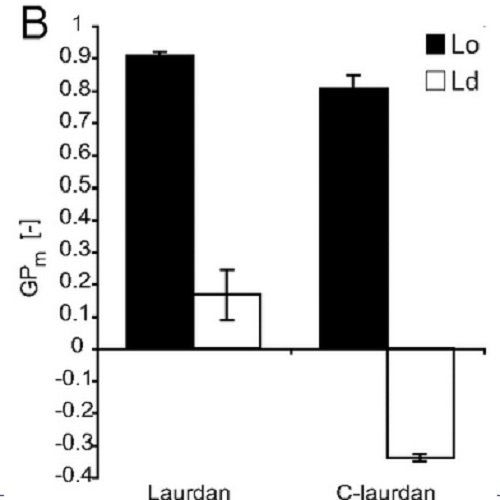
Lipid rafts are nanoscopic assemblies of sphingolipids, cholesterol, and specific membrane proteins that contribute to lateral heterogeneity in eukaryotic membranes. Separation of artificial membranes into liquid-ordered (Lo) and liquid-disordered phases is regarded as a common model for this compartmentalization. However, tight lipid packing in Lo phases seems to conflict with efficient partitioning of raft-associated transmembrane (TM) proteins. To assess membrane order as a component of raft organization, we performed fluorescence spectroscopy and microscopy with the membrane probes Laurdan and C-laurdan. First, we assessed lipid packing in model membranes of various compositions and found cholesterol and acyl chain dependence of membrane order. Then we probed cell membranes by using two novel systems that exhibit inducible phase separation: giant plasma membrane vesicles [Baumgart et al. (2007) Proc Natl Acad Sci USA 104:3165-3170] and plasma membrane spheres. Notably, only the latter support selective inclusion of raft TM proteins with the ganglioside GM1 into one phase. We measured comparable small differences in order between the separated phases of both biomembranes. Lateral packing in the ordered phase of giant plasma membrane vesicles resembled the Lo domain of model membranes, whereas the GM1 phase in plasma membrane spheres exhibited considerably lower order, consistent with different partitioning of lipid and TM protein markers. Thus, lipid-mediated coalescence of the GM1 raft domain seems to be distinct from the formation of a Lo phase, suggesting additional interactions between proteins and lipids to be effective.
- Proc. Natl. Acad. Sci. U.S.A. 2009 Sep 29;106(39):16645-50
- 2009
- Cell Biology
- 19805351
- PubMed
Enabled by:
