Dynamic coupling of pattern formation and morphogenesis in the developing vertebrate retina.
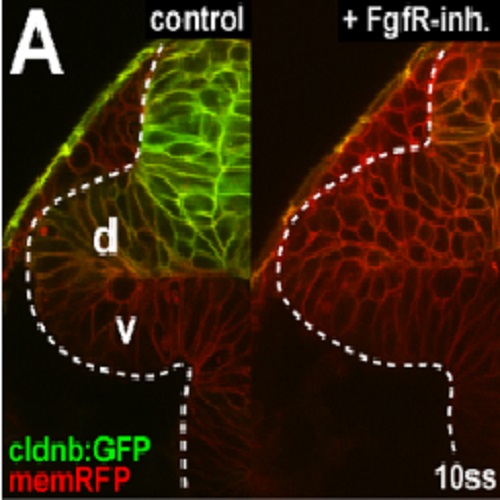
During embryonic development, pattern formation must be tightly synchronized with tissue morphogenesis to coordinate the establishment of the spatial identities of cells with their movements. In the vertebrate retina, patterning along the dorsal-ventral and nasal-temporal (anterior-posterior) axes is required for correct spatial representation in the retinotectal map. However, it is unknown how specification of axial cell positions in the retina occurs during the complex process of early eye morphogenesis. Studying zebrafish embryos, we show that morphogenetic tissue rearrangements during eye evagination result in progenitor cells in the nasal half of the retina primordium being brought into proximity to the sources of three fibroblast growth factors, Fgf8/3/24, outside the eye. Triple-mutant analysis shows that this combined Fgf signal fully controls nasal retina identity by regulating the nasal transcription factor Foxg1. Surprisingly, nasal-temporal axis specification occurs very early along the dorsal-ventral axis of the evaginating eye. By in vivo imaging GFP-tagged retinal progenitor cells, we find that subsequent eye morphogenesis requires gradual tissue compaction in the nasal half and directed cell movements into the temporal half of the retina. Balancing these processes drives the progressive alignment of the nasal-temporal retina axis with the anterior-posterior body axis and is controlled by a feed-forward effect of Fgf signaling on Foxg1-mediated cell cohesion. Thus, the mechanistic coupling and dynamic synchronization of tissue patterning with morphogenetic cell behavior through Fgf signaling leads to the graded allocation of cell positional identity in the eye, underlying retinotectal map formation.
- PLoS Biol. 2009 Oct 13;7(10):e1000214
- 2009
- Developmental Biology
- 19823566
- PubMed
Enabled by:
