Improvement of ultrastructural preservation of Eimeria oocysts by microwave-assisted chemical fixation or by high pressure freezing and freeze substitution.
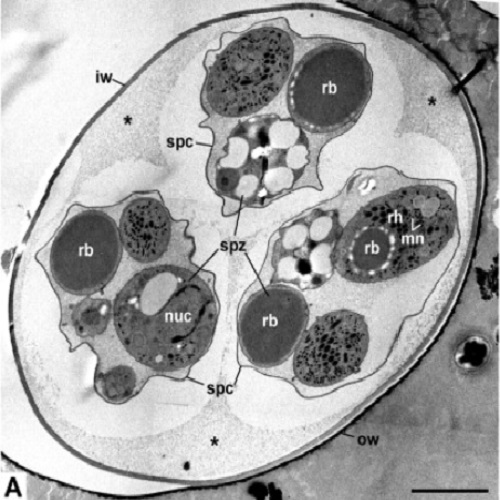
Fixation and preparation for electron microscopy of coccidian oocysts is a general problem. Especially in sporulated oocysts, proper fixation and resin infiltration are hindered by the robust oocyst wall. Conventional chemical fixation therefore leads to artefacts that obscure cellular details in the oocysts. In this study, sporulated oocysts of Eimeria nieschulzi were subjected to different fixation and embedding protocols: conventional chemical fixation and embedding in Spurr's resin, microwave-assisted fixation and processing followed by embedding in epon, and high pressure freezing followed by freeze substitution and epon embedding. The samples were finally studied by transmission electron microscopy. Many ultrastructural features of the oocyst wall and the sporozoites were already substantially improved after microwaved-assisted fixation and processing. However, the fine structural preservation still suffered from shrinkage and artificial extraction, which occured during dehydration and infiltration. High pressure freezing (HPF) and freeze substitution (FS) revealed much better preservation. Oocyst walls retained their ovoid shape, and the ultrastructure of sporozoites was well preserved with no signs of shrinkage or extraction. HPF and FS are therefore a suitable method for the ultrastructural analysis of coccidian oocysts.
- Protist 2012 Mar 20;163(2):296-305
- 2012
- Imaging Technologies Development
- 21764370
- PubMed
Enabled by:
