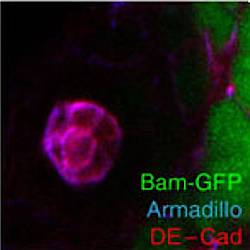
Local BMP receptor activation at adherens junctions in the Drosophila germline stem cell niche.
According to the stem cell niche synapse hypothesis postulated for the mammalian haematopoietic system, spatial specificity of niche signals is maximized by subcellularly restricting signalling to cadherin-based adherens junctions between individual niche and stem cells. However, such a synapse has never been observed directly, in part, because tools to detect active growth factor receptors with subcellular resolution were not available. Here we describe a novel fluorescence-based reporter that directly visualizes bone morphogenetic protein (BMP) receptor activation and show that in the Drosophila testis a BMP niche signal is transmitted preferentially at adherens junctions between hub and germline stem cells, resembling the proposed synapse organization. Ligand secretion involves the exocyst complex and the Rap activator Gef26, both of which are also required for Cadherin trafficking towards adherens junctions. We, therefore, propose that local generation of the BMP signal is achieved through shared use of the Cadherin transport machinery.
Back to list