Polar actomyosin contractility destabilizes the position of the cytokinetic furrow.
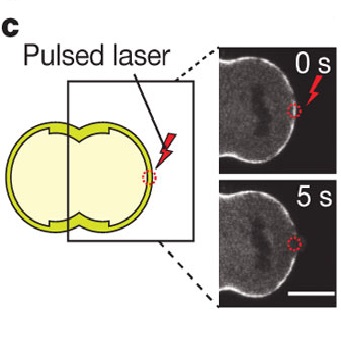
Cytokinesis, the physical separation of daughter cells at the end of mitosis, requires precise regulation of the mechanical properties of the cell periphery. Although studies of cytokinetic mechanics mostly focus on the equatorial constriction ring, a contractile actomyosin cortex is also present at the poles of dividing cells. Whether polar forces influence cytokinetic cell shape and furrow positioning remains an open question. Here we demonstrate that the polar cortex makes cytokinesis inherently unstable. We show that limited asymmetric polar contractions occur during cytokinesis, and that perturbing the polar cortex leads to cell shape oscillations, resulting in furrow displacement and aneuploidy. A theoretical model based on a competition between cortex turnover and contraction dynamics accurately accounts for the oscillations. We further propose that membrane blebs, which commonly form at the poles of dividing cells and whose role in cytokinesis has long been enigmatic, stabilize cell shape by acting as valves releasing cortical contractility. Our findings reveal an inherent instability in the shape of the dividing cell and unveil a novel, spindle-independent mechanism ensuring the stability of cleavage furrow positioning.
- Nature 2011 Aug 25;476(7361):462-6
- 2011
- Cell Biology
- 21822289
- PubMed
Enabled by:
