Dynein motion switches from diffusive to directed upon cortical anchoring.
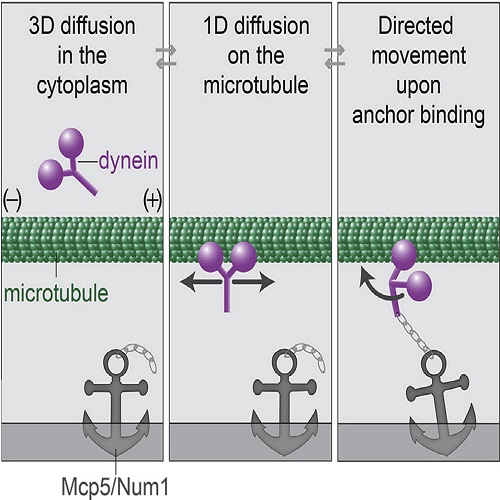
Cytoplasmic dynein is a motor protein that exerts force on microtubules. To generate force for the movement of large organelles, dynein needs to be anchored, with the anchoring sites being typically located at the cell cortex. However, the mechanism by which dyneins target sites where they can generate large collective forces is unknown. Here, we directly observe single dyneins during meiotic nuclear oscillations in fission yeast and identify the steps of the dynein binding process: from the cytoplasm to the microtubule and from the microtubule to cortical anchors. We observed that dyneins on the microtubule move either in a diffusive or directed manner, with the switch from diffusion to directed movement occurring upon binding of dynein to cortical anchors. This dual behavior of dynein on the microtubule, together with the two steps of binding, enables dyneins to self-organize into a spatial pattern needed for them to generate large collective forces.
- Cell 2013 Jun 20;153(7):1526-36
- 2013
- Cell Biology
- 23791180
- PubMed
Enabled by:
