The nuclear cap-binding complex interacts with the U4/U6·U5 tri-snRNP and promotes spliceosome assembly in mammalian cells.
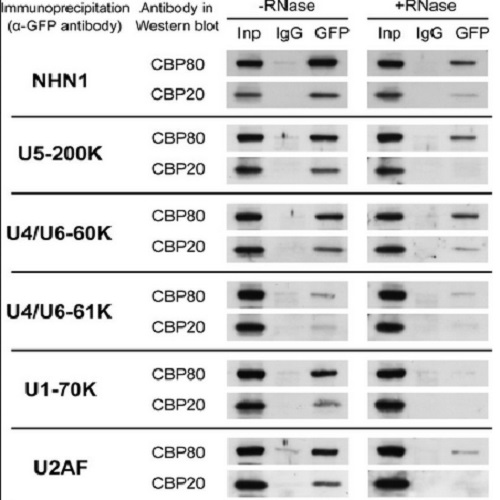
The nuclear cap-binding complex (CBC) binds to the 7-methyl guanosine cap present on every RNA polymerase II transcript. CBC has been implicated in many aspects of RNA biogenesis; in addition to roles in miRNA biogenesis, nonsense-mediated decay, 3'-end formation, and snRNA export from the nucleus, CBC promotes pre-mRNA splicing. An unresolved question is how CBC participates in splicing. To investigate CBC's role in splicing, we used mass spectrometry to identify proteins that copurify with mammalian CBC. Numerous components of spliceosomal snRNPs were specifically detected. Among these, three U4/U6·U5 snRNP proteins (hBrr2, hPrp4, and hPrp31) copurified with CBC in an RNA-independent fashion, suggesting that a significant fraction of CBC forms a complex with the U4/U6·U5 snRNP and that the activity of CBC might be associated with snRNP recruitment to pre-mRNA. To test this possibility, CBC was depleted from HeLa cells by RNAi. Chromatin immunoprecipitation and live-cell imaging assays revealed decreased cotranscriptional accumulation of U4/U6·U5 snRNPs on active transcription units, consistent with a requirement for CBC in cotranscriptional spliceosome assembly. Surprisingly, recruitment of U1 and U2 snRNPs was also affected, indicating that RNA-mediated interactions between CBC and snRNPs contribute to splicing. On the other hand, CBC depletion did not impair snRNP biogenesis, ruling out the possibility that decreased snRNP recruitment was due to changes in nuclear snRNP concentration. Taken together, the data support a model whereby CBC promotes pre-mRNA splicing through a network of interactions with and among spliceosomal snRNPs during cotranscriptional spliceosome assembly.
- RNA 2013 Aug 21;19(8):1054-63
- 2013
- Cell Biology
- 23793891
- PubMed
Enabled by:
