Microtubule polarity predicts direction of egg chamber rotation in Drosophila.
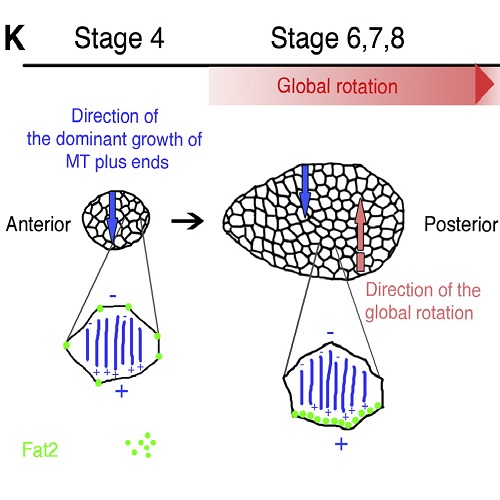
Whole-tissue rotations have recently been recognized as a widespread morphogenetic process important for tissue elongation [1-4]. In Drosophila ovaries, elongation of the egg chamber involves a global rotation of the follicle epithelium along the anterior-posterior axis [5]. Individual egg chambers rotate either in a clockwise or counterclockwise direction; however, how the symmetry of egg chambers is broken to allow rotation remains unknown. Here we show that at the basal side of follicle cells, microtubules are preferentially aligned perpendicular to the anterior-posterior axis of the egg chamber. Microtubule depolymerization stalls egg chamber rotation and egg chamber elongation. The preferential alignment of microtubules and egg chamber rotation depend on the atypical cadherin Fat2 and the planar polarized Fat2 localization depends on intact microtubules. Moreover, by tracking microtubule plus-end growth in vivo using EB1::GFP, we find that microtubules are highly polarized in the plane of the follicle epithelium. Polarization of microtubules precedes the onset of egg chamber rotation and predicts the direction of rotation. Our data suggest a feedback amplification mechanism between Fat2 localization and microtubule polarity involved in breaking symmetry and directing egg chamber rotation.
- Curr. Biol. 2013 Aug 5;23(15):1472-7
- 2013
- Developmental Biology
- 23831293
- PubMed
Enabled by:
