Monitoring actin cortex thickness in live cells.
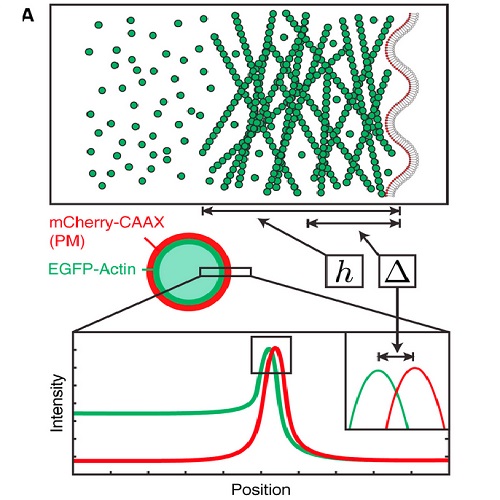
Animal cell shape is controlled primarily by the actomyosin cortex, a thin cytoskeletal network that lies directly beneath the plasma membrane. The cortex regulates cell morphology by controlling cellular mechanical properties, which are determined by network structure and geometry. In particular, cortex thickness is expected to influence cell mechanics. However, cortex thickness is near the resolution limit of the light microscope, making studies relating cortex thickness and cell shape challenging. To overcome this, we developed an assay to measure cortex thickness in live cells, combining confocal imaging and subresolution image analysis. We labeled the actin cortex and plasma membrane with chromatically different fluorophores and measured the distance between the resulting intensity peaks. Using a theoretical description of cortex geometry and microscopic imaging, we extracted an average cortex thickness of ?190 nm in mitotic HeLa cells and tested the validity of our assay using cell images generated in silico. We found that thickness increased after experimental treatments preventing F-actin disassembly. Finally, we monitored physiological changes in cortex thickness in real-time during actin cortex regrowth in cellular blebs. Our investigation paves the way to understanding how molecular processes modulate cortex structure, which in turn drives cell morphogenesis.
- Biophys. J. 2013 Aug 6;105(3):570-80
- 2013
- Biophysics
- 23931305
- PubMed
Enabled by:
