Asymmetric supported lipid bilayer formation via methyl-β-cyclodextrin mediated lipid exchange: influence of asymmetry on lipid dynamics and phase behavior.
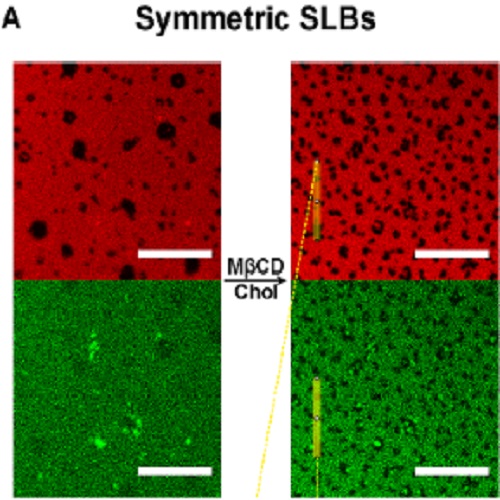
Supported lipid bilayers (SLBs) are broadly used as minimal membrane models and commonly produced by vesicle fusion (VF) on solid supports. Despite its advantages, VF does not allow the controlled formation of bilayers that mimic the leaflet asymmetry in lipid composition normally found in biological systems. Here we present a simple, quick, and versatile method to produce SLBs with a desired asymmetric lipid composition which is stable for ca. 4 h. We apply methyl-β-cyclodextrin mediated lipid exchange to SLBs formed by VF to enrich the upper leaflet of the bilayer with sphingomyelin. The bilayer asymmetry is assessed by fluorescence correlation spectroscopy, measuring the lipid mobility separately in each leaflet. To check the compatibility of the method with the most common protein reconstitution approaches, we report the production of asymmetric SLBs (aSLBs) in the presence of a glycosylphosphatidylinositol-anchored protein, reconstituted in the bilayer both, via direct protein insertion, and via proteoliposomes fusion. We finally apply aSLBs to study phase separation and transbilayer lipid movement of raft-mimicking lipid mixtures. The observed differences in terms of phase separation in symmetric and asymmetric SLBs with the same overall lipid composition provide further experimental evidence that the transversal lipid distribution affects the overall lipid miscibility and allow to temporally investigate leaflet mixing.
Back to list