Local increases in mechanical tension shape compartment boundaries by biasing cell intercalations.
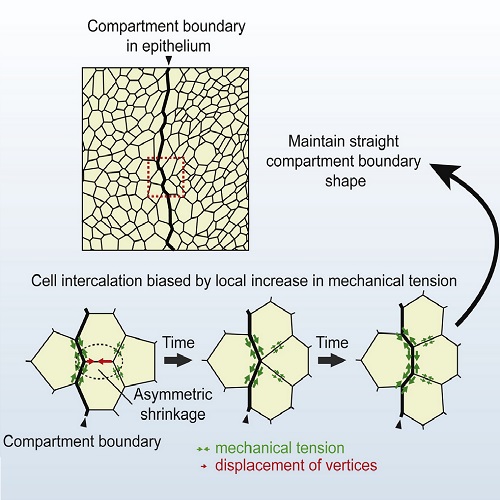
Mechanical forces play important roles during tissue organization in developing animals. Many tissues are organized into adjacent, nonmixing groups of cells termed compartments. Boundaries between compartments display a straight morphology and are associated with signaling centers that are important for tissue growth and patterning. Local increases in mechanical tension at cell junctions along compartment boundaries have recently been shown to prevent cell mixing and to maintain straight boundaries. The cellular mechanisms by which local increases in mechanical tension prevent cell mixing at compartment boundaries, however, remain poorly understood. Here, we have used live imaging and quantitative image analysis to determine cellular dynamics at and near the anteroposterior compartment boundaries of the Drosophila pupal abdominal epidermis. We show that cell mixing within compartments involves multiple cell intercalations. Frequency and orientation of cell intercalations are unchanged along the compartment boundaries; rather, an asymmetry in the shrinkage of junctions during intercalation is biased, resulting in cell rearrangements that suppress cell mixing. Simulations of tissue growth show that local increases in mechanical tension can account for this bias in junctional shrinkage. We conclude that local increases in mechanical tension maintain cell populations separate by influencing junctional rearrangements during cell intercalation.
- Curr. Biol. 2014 Aug 4;24(15):1798-805
- 2014
- Biophysics
- 25065753
- PubMed
Enabled by:
