Daylight vision repair by cell transplantation.
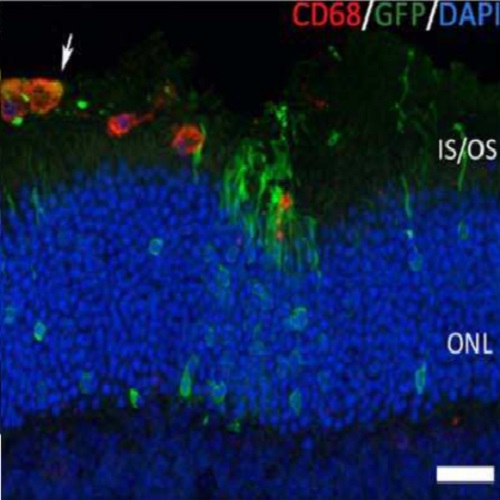
Human daylight vision depends on cone photoreceptors and their degeneration results in visual impairment and blindness as observed in several eye diseases including age-related macular degeneration, cone-rod dystrophies, or late stage retinitis pigmentosa, with no cure available. Preclinical cell replacement approaches in mouse retina have been focusing on rod dystrophies, due to the availability of sufficient donor material from the rod-dominated mouse retina, leaving the development of treatment options for cone degenerations not well studied. Thus, an abundant and traceable source for donor cone-like photoreceptors was generated by crossing neural retina leucine zipper-deficient (Nrl(-/-) ) mice with an ubiquitous green fluorescent protein (GFP) reporter line resulting in double transgenic tg(Nrl(-/-); aGFP) mice. In Nrl(-/-) retinas, all rods are converted into cone-like photoreceptors that express CD73 allowing their enrichment by CD73-based magnetic activated cell sorting prior transplantation into the subretinal space of adult wild-type, cone-only (Nrl(-/-)), or cone photoreceptor function loss 1 (Cpfl1) mice. Donor cells correctly integrated into host retinas, acquired mature photoreceptor morphology, expressed cone-specific markers, and survived for up to 6 months, with significantly increased integration rates in the cone-only Nrl(-/-) retina. Individual retinal ganglion cell recordings demonstrated the restoration of photopic responses in cone degeneration mice following transplantation suggesting, for the first time, the feasibility of daylight vision repair by cell replacement in the adult mammalian retina.
- Stem Cells 2015 Jan;33(1):79-90
- 2015
- Cell Biology
- 25183393
- PubMed
Enabled by:
