Essential role of endocytosis for interleukin-4-receptor-mediated JAK/STAT signalling.
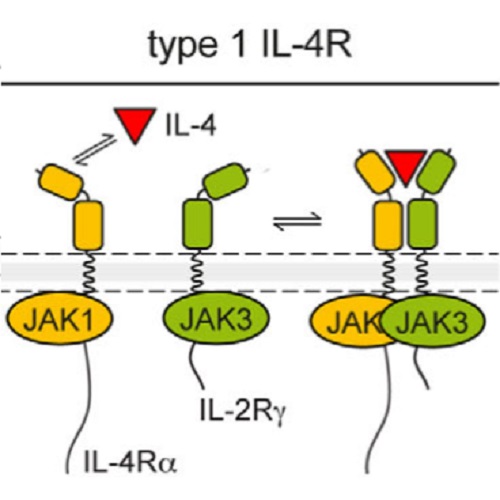
Many important signalling cascades operate through specialized signalling endosomes, but a corresponding mechanism has as yet not been described for hematopoietic cytokine receptors. Based on live-cell affinity measurements, we recently proposed that ligand-induced interleukin-4 receptor (IL-4R) complex formation and thus JAK/STAT pathway activation requires a local subcellular increase in receptor density. Here, we show that this concentration step is provided by the internalization of IL-4R subunits through a constitutive, Rac1-, Pak- and actin-mediated endocytosis route that causes IL-4R subunits to become enriched by about two orders of magnitude within a population of cortical endosomes. Consistently, ligand-induced receptor dimers are preferentially detected within these endosomes. IL-4 signalling can be blocked by pharmacological inhibitors targeting the actin polymerization machinery driving receptor internalization, placing endocytosis unambigously upstream of receptor activation. Taken together, these observations demonstrate a role for endocytosis that is mechanistically distinct from the scaffolding function of signalling endosomes in other pathways.
- J. Cell. Sci. 2015 Oct 15;128(20):3781-95
- 2015
- Cell Biology
- 26306492
- PubMed
Enabled by:
