Oxygen Tension Within the Neurogenic Niche Regulates Dopaminergic Neurogenesis in the Developing Midbrain.
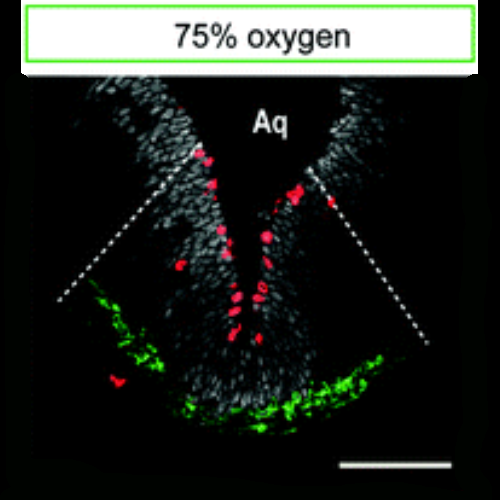
Oxygen tension is an important factor controlling stem cell proliferation and maintenance in various stem cell populations with a particular relevance in midbrain dopaminergic progenitors. Further studies have shown that the oxygen-dependent transcription factor hypoxia-inducible factor 1alpha (HIF-1alpha) is involved in these processes. However, all available studies on oxygen effects in dopaminergic neuroprogenitors were performed in vitro and thus it remains unclear whether tissue oxygen tension in the embryonic midbrain is also relevant for the regulation of dopaminergic neurogenesis in vivo. We thus dissect here the effects of oxygen tension in combination with HIF-1alpha conditional knockout on dopaminergic neurogenesis by using a novel experimental design allowing for the control of oxygen tension within the microenvironment of the neurogenic niche of the murine fetal midbrain in vivo. The microenvironment of the midbrain dopaminergic neurogenic niche was detected as hypoxic with oxygen tensions below 1.1%. Maternal oxygen treatment of 10%, 21%, and 75% atmospheric oxygen tension for 48 h translates into robust changes in fetal midbrain oxygenation. Fetal midbrain hypoxia hampered the generation of dopaminergic neurons and is accompanied with restricted fetal midbrain development. In contrast, induced hyperoxia stimulated proliferation and differentiation of dopaminergic progenitors during early and late embryogenesis. Oxygen effects were not directly mediated through HIF-1alpha signaling. These data--in agreement with in vitro data-indicate that oxygen is a crucial regulator of developmental dopaminergic neurogenesis. Our study provides the initial framework for future studies on molecular mechanisms mediating oxygen regulation of dopaminergic neurogenesis within the fetal midbrain as its natural environment.
Back to list