Green bioprinting: extrusion-based fabrication of plant cell-laden biopolymer hydrogel scaffolds.
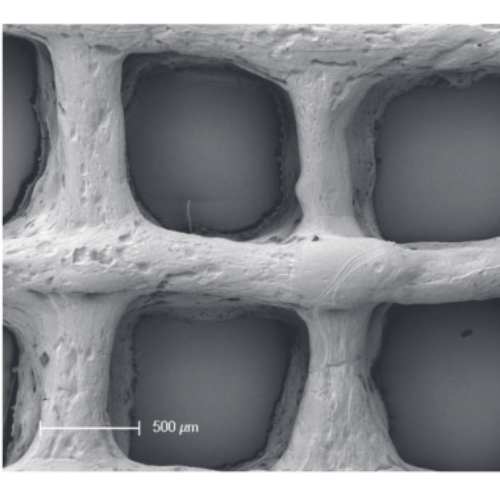
Plant cell cultures produce active agents for pharmaceuticals, food and cosmetics. However, up to now process control for plant cell suspension cultures is challenging. A positive impact of cell immobilization, such as encapsulation in hydrogel beads, on secondary metabolites production has been reported for several plant species. The aim of this work was to develop a method for bioprinting of plant cells in order to allow fabrication of free-formed three-dimensional matrices with defined internal pore architecture for in depth characterization of immobilization conditions, cell agglomeration and interactions. By using extrusion-based 3D plotting of a basil cell-laden hydrogel blend consisting of alginate, agarose and methylcellulose (alg/aga/mc), we could demonstrate that bioprinting is applicable to plant cells. The majority of the cells survived plotting and crosslinking and the embedded cells showed high viability and metabolic activity during the investigated cultivation period of 20 d. Beside its compatibility with the plant cells, the novel alg/aga/mc blend allowed fabrication of defined 3D constructs with open macropores both in vertical and horizontal direction which were stable under culture conditions for several weeks. Thus, Green Bioprinting, an additive manufacturing technology processing live cells from the plant kingdom, is a promising new immobilization tool for plant cells that enables the development of new bioprocesses for secondary metabolites production as well as monitoring methods.
- Biofabrication. 2017 Nov 14;9(4):045011
- 2017
- Biomaterials
- 28837040
- PubMed
Enabled by:
