Local Nucleation of Microtubule Bundles through Tubulin Concentration into a Condensed Tau Phase.
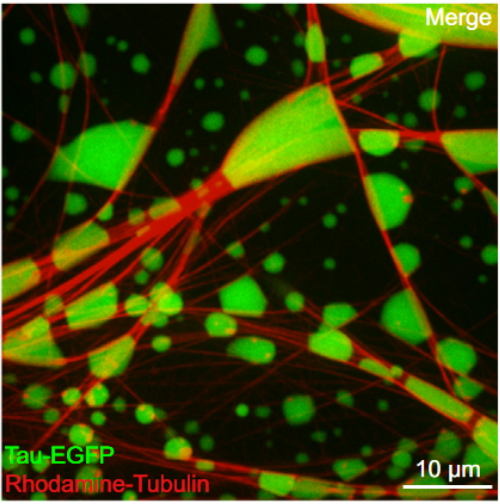
Non-centrosomal microtubule bundles play important roles in cellular organization and function. Although many diverse proteins are known that can bundle microtubules, biochemical mechanisms by which cells could locally control the nucleation and formation of microtubule bundles are understudied. Here, we demonstrate that the concentration of tubulin into a condensed, liquid-like compartment composed of the unstructured neuronal protein tau is sufficient to nucleate microtubule bundles. We show that, under conditions of macro-molecular crowding, tau forms liquid-like drops. Tubulin partitions into these drops, efficiently increasing tubulin concentration and driving the nucleation of microtubules. These growing microtubules form bundles, which deform the drops while remaining enclosed by diffusible tau molecules exhibiting a liquid-like behavior. Our data suggest that condensed compartments of microtubule bundling proteins could promote the local formation of microtubule bundles in neurons by acting as non-centrosomal microtubule nucleation centers and that liquid-like tau encapsulation could provide both stability and plasticity to long axonal microtubule bundles.
- Cell Rep. 2017 Sep 5;20(10):2304-2312
- 2017
- Cell Biology
- 28877466
- PubMed
Enabled by:
