VAMP-associated protein-A and oxysterol-binding protein-related protein 3 promote the entry of late endosomes into the nucleoplasmic reticulum.
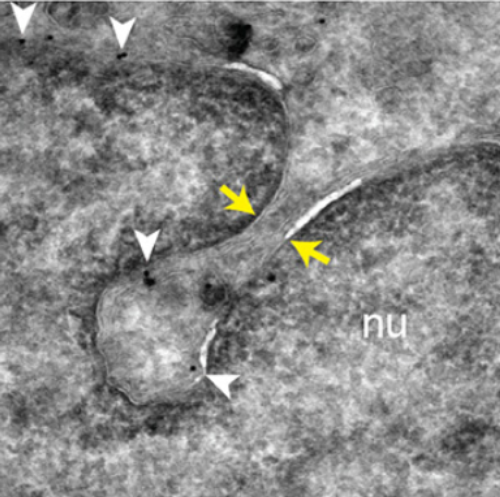
The endocytic pathway plays an instrumental role in recycling internalized molecules back to the plasma membrane or in directing them to lysosomes for degradation. We recently reported a new role of endosomes-the delivery of components from extracellular vesicles (EVs) to the nucleoplasm of recipient cells. Using indirect immunofluorescence, FRET, immunoisolation techniques, and RNAi, we report here a tripartite protein complex (referred to as the VOR complex) that is essential for the nuclear transfer of EV-derived components by orchestrating the specific localization of late endosomes into nucleoplasmic reticulum. We found that the VOR complex contains the endoplasmic reticulum-localized vesicle-associated membrane protein (VAMP)-associated protein A (VAP-A), the cytoplasmic oxysterol-binding protein-related protein 3 (ORP3), and late endosome-associated small GTPase Rab7. The silencing of VAP-A or ORP3 abrogated the association of Rab7-positive late endosomes with nuclear envelope invaginations and, hence, the transport of endocytosed EV-derived components to the nucleoplasm of recipient cells. We conclude that the VOR complex can be targeted to inhibit EV-mediated intercellular communication, which can have therapeutic potential for managing cancer in which the release of EVs is dysregulated.
- J Biol Chem. 2018 Sep 7;293(36):13834-13848
- 2018
- Cell Biology
- 30018135
- PubMed
Enabled by:
